BN-Substituted coronene diimide donor–acceptor–donor triads: photophysical, (spectro)-electrochemical studies and Lewis behavior†
Abstract
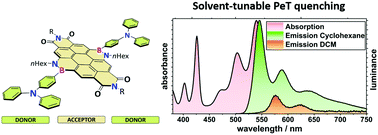
Boron/nitrogen substituted polyaromatic hydrocarbons (PAHs) are unique materials, with similar molecular structures to their carbon/carbon analogs, but different electronic properties. We report how these may be tuned by substitution at the B and/or N atoms: the BN-PAH analogues we investigated are BN-substituted coronene diimide (BNCDI) acceptors, combined with electron-rich (hetero)arene substituents (donors) on the boron atoms at either side of the BNCDI, resulting in donor–acceptor–donor (D–A–D) triads. In comparison to the all-carbon coronene diimide, the implementation of two BN units led to a bathochromic shift of absorption/emission (44 nm) and luminescence quantum yields close to unity. The uniqueness of the BN-based D–A–D motif became clear as the substitution effect of the (hetero)arene substituents was investigated. The strong electron-donating triphenylamine motif had a minor influence on the absorption behavior but showed strongly solvent-dependent luminescence properties. This could be attributed to an intramolecular photoinduced electron transfer (PeT) process which was supported by a Rehm–Weller analysis and density functional theory computations. This process was not observed for the other substituents. Moreover, we probed the influence of the aryl substituent on the B–N bond itself by using (spectro)electrochemistry and analyzed the Lewis behavior of the BN unit. The species that formed showed strong absorptions across the whole UV/vis/NIR region.
- This article is part of the themed collection: 2021 Journal of Materials Chemistry C most popular articles


 Please wait while we load your content...
Please wait while we load your content...