End-functionalization of dithiarubicene: modulation of optoelectronic properties by metal-catalyzed coupling reactions and device application†
Abstract
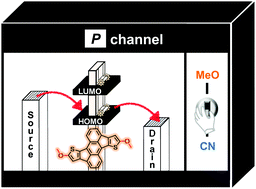
In order to modulate the optoelectronic properties of dithiarubicene (DTR) by end-functionalization and reveal the structure–property relationship in detail, we have carried out a set of metal-catalyzed coupling reactions. Electron-withdrawing cyano (CN-DTR) and pyridyl (Py-DTR) groups, electron-donating methoxy (MeO-DTR) and thienyl (Th-DTR) groups, and relatively electron-neutral trimethylsilylethynyl (Ethynyl-DTR) group were introduced on the DTR core by using halogenated DTR derivatives as precursors. Particularly, we could successfully introduce the methoxy group by CuO-catalyzed reactions in high yield. As a result of the combination of the electron-accepting DTR core and the electron-donating methoxy group, the UV-Vis-NIR absorption spectrum of MeO-DTR showed a large red-shift of the peak maximum by approximately 90 nm. In addition, the introduction of substituents made it possible to finely tune the HOMO and LUMO energy levels of DTR thereby enabling its application in OFET devices. Thanks to the elevated HOMO energy level, the OFET device based on MeO-DTR exhibited a hole mobility (μh) of up to 1.4 × 10−3 cm2 V−1 s−1. On the other hand, the device based on CN-DTR had an ambipolar characteristic, which exhibited a moderate electron mobility (μe = 1.1 × 10−3 cm2 V−1 s−1) due to its low-lying LUMO energy level. From the XRD measurements, MeO-DTR and CN-DTR were found to adopt an edge-on molecular orientation on the HMDS-treated SiO2 substrate.


 Please wait while we load your content...
Please wait while we load your content...