Two-step assembly kinetics of gold nanoparticles†
Abstract
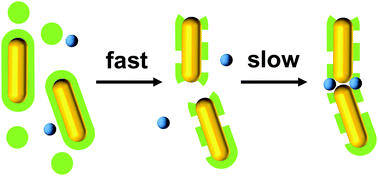
We study the assembly kinetics of surfactant-stabilized gold nanoparticles in the presence of sulfate ions. The reaction proceeds in two steps: very rapid (a few minutes) formation of amorphous aggregates, followed by slow reordering (over several hours). The latter process is the only one detectable via absorbance spectroscopy and results in the formation of intimate contacts between the objects, with interparticle distances below the thickness of a surfactant bilayer. The rate-limiting step of the reaction could be related to surfactant expulsion from the initial aggregates, which allows the particles to come in close contact and form chains. There are marked differences in reaction yield and rate constant between spheres, rods and bipyramids, highlighting the role of surface curvature in contact formation. Once formed, the assemblies are very sturdy and stable under centrifugation and dialysis. The contact interaction is strong and highly directional, as shown by liquid-cell transmission electron microscopy.


 Please wait while we load your content...
Please wait while we load your content...