Mechanistic investigation of redox processes in Zn–MnO2 battery in mild aqueous electrolytes†
Abstract
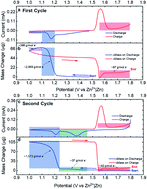
Zinc–MnO2 based batteries have acquired attention for grid-level applications, due to impressive theoretical performance, cost effectiveness and intrinsic safety. However, there are still many challenges that remain elusive due to the complex and controversial mechanisms of operation that hinder commercialization. In this work, the detailed redox processes that occur at the cathode during Zn–MnO2 battery operation are elucidated. Using a blend of structural and electrochemical techniques, the redox pairs that occur during operation are mechanistically studied while also showcasing the true impact of the electrolyte additive (0.1 M MnSO4) in a 1 M ZnSO4 electrolyte. An electrochemical quartz-crystal microbalance (EQCM) has been leveraged to reveal the effect of zinc hydroxy sulfate salt (Zn4SO4(OH)6·nH2O) and zinc manganese oxide (ZnxMnyOz) dissolution/deposition, which are believed to be major components during discharge and charge conditions. These results provide insight not currently available, allowing a holistic view of the electrochemical reaction mechanisms during battery operation.


 Please wait while we load your content...
Please wait while we load your content...