Insight into the synthesis and tuning of uncoated, core–shell structured lithium nickel phosphate†
Abstract
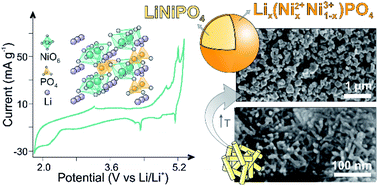
The emerging market of high voltage electronics signified the importance of the development of novel cathodes with high operating potentials. Lithium nickel phosphate (LNP), a suitable candidate with an operating potential of 5.1 V vs. Li/Li+, is however presented with a number of challenges. In this respect, economically synthesizing nanoparticles in a certain phase with a narrow size distribution to shorten the Li+ diffusion pathways is desired. The polyol-mediated method reported here provides a novel insight into low-temperature synthesis of olivine-type cathodes with deliberate tuning of the surface chemistry, morphology and product composition. The thereby produced LNP indeed demonstrated high electrochemical activity without any carbon coating or doping, correlating to more than electrolyte decomposition and/or side reactions. The well-defined de-/intercalation of the Li+ occurred at high potentials of 4.9 V (Ni2+/Ni3+) and 5.1 V (Ni3+/Ni4+). The significance of the operating conditions was evidenced by the enhanced electrochemical performance when the identified kinetic limitations were considered in the adopted cycling strategy. A high specific capacity of 85 mA h g−1 was thereby delivered, reaching an energy density of 207 W h kg−1.


 Please wait while we load your content...
Please wait while we load your content...