The efficacy of Lewis affinity scale metrics to represent solvent interactions with reagent salts in all-inorganic metal halide perovskite solutions†
Abstract
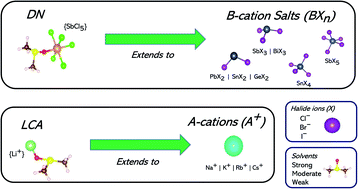
Solvents employed in the solution processing of metal halide perovskites are known to play a key role in defining the morphology and properties of the resulting thin film, and thus the performance of perovskite solar cell devices. Accurate metrics are needed that are capable of differentiating among candidates, finding solvents that adequately solubilize the various precursor species in solution and facilitate the nucleation and growth of these materials. Existing metrics such as the unsaturated Mayer bond order (UMBO) and the Gutmann donor number (DN) have been tested for lead iodide perovskite systems; but there has yet to be a comprehensive study on their transferability to lead-free perovskite solutions. We use ab initio methods (density functional theory) and regression analysis tools to study the usefulness of DN and BF3 affinity scales in this regard. We compared the relative effectiveness of these scales to describe interactions between solvents and BXn perovskite salts of lead (Pb2+), tin (Sn2+ and Sn4+), germanium (Ge2+), bismuth (Bi3+), and antimony (Sb3+ and Sb5+). The DN proved to be a better representation than the BF3 of such interactions, reflecting the closer similarity of these species to the “parent” SbCl5 Lewis acid than to BF3. In addition, we have uncovered the usefulness of the lithium cation affinity metric (LCA) to describe the strength of interactions between solvents and A-site cations (e.g. Na+, K+, Rb+ and Cs+) in all-inorganic metal halide perovskite solutions. We find that the coordination strengths of solvents towards species in all-inorganic metal halide perovskite solutions are best described by two different metrics with distinct modes of action: DN differentiates among BXn salt complexes, and LCA among A-site cation species. This revelation can help guide the choice of solvent to optimize processing conditions. It also emphasizes the importance of selecting solvents whose DN and LCA optimize coordination to key Lewis acid species in all-inorganic perovskite solutions.
- This article is part of the themed collection: Editor’s Choice: Perovskite-based solar cells


 Please wait while we load your content...
Please wait while we load your content...