Mechanochemically synthesized Pb-free halide perovskite-based Cs2AgBiBr6–Cu–RGO nanocomposite for photocatalytic CO2 reduction†
Abstract
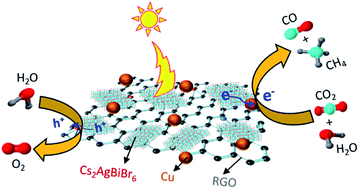
Pb-based halide perovskites have recently showed great potential in various applications such as solar cells, optoelectronics and photocatalysis. Despite their high performance, the Pb2+ toxicity along with poor stability hinders long term applications in photocatalysis. Herein, we report mechanochemically prepared Pb-free Cs2AgBiBr6 double perovskite nanoplates and their heterostructure with Cu-loaded reduced graphene oxide (Cu–RGO) for gas-phase photocatalytic CO2 reduction using water vapor as the proton source in the absence of a hole scavenger. The resulting Cs2AgBiBr6–Cu–RGO nanocomposite shows significant photocatalytic activity of 10.7 (±0.6) μmol CH4 g−1 h−1, 1.9 (±0.3) μmol CO g−1 h−1 and 1.0 (±0.2) μmol H2 g−1 h−1, with a CH4 selectivity of 93.0 (±0.5)% on an electron basis with 1 sun and a remarkable apparent quantum efficiency of 0.89 (±0.21)% at 590 nm. A further 32% enhancement in photocatalytic activity on an electron basis is achieved when the light intensity is doubled (2 suns). The high performance was attributed to their improved charge separation and suppressed electron–hole recombination, along with extended visible light absorption, better stability in a humid environment and improved CO2 adsorption. These findings support Cs2AgBiBr6 as a potential Pb-free alternative to conventional halide perovskites for photocatalytic solar-to-fuel conversion and CO2 utilization.


 Please wait while we load your content...
Please wait while we load your content...