A rigorous electrochemical ammonia electrolysis protocol with in operando quantitative analysis†
Abstract
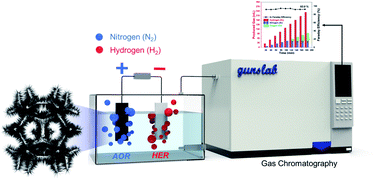
Ammonia has emerged as an attractive liquid fuel for hydrogen production owing to its facile transportation, high capacity of hydrogen storage, and ecofriendly environmental products (N2 and H2). Moreover, the electrolysis of ammonia to produce nitrogen and hydrogen only requires an external voltage of 0.06 V theoretically, which is much lower than the energy needed for water electrolysis (1.23 V). In this study, we propose a well-established procedure using in operando gas chromatography that enables us to reliably compare and evaluate the new catalyst for ammonia oxidation. With the protocol, we could distinguish in detail the competitive oxidation reaction between the ammonia oxidation and oxygen evolution reactions with real-time monitoring. Using a flower-like electrodeposited Pt catalyst, we have efficiently produced hydrogen with less power consumption of 734 LH2 kW h−1, which is significantly lower than that of the water-splitting process (242 LH2 kW h−1). The use of this rigorous protocol should help to evaluate the practical performances for ammonia oxidation, thus enabling the field to focus on viable pathways towards the practical electrochemical oxidation of ammonia to hydrogen.


 Please wait while we load your content...
Please wait while we load your content...