Revealing the role of defects in graphene oxide in the evolution of magnesium nanocrystals and the resulting effects on hydrogen storage†
Abstract
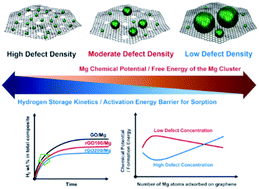
The hydrogen storage properties of magnesium (Mg) considerably rely on the size and morphology of Mg particles that determine the diffusion path for hydrogen atoms. Despite the intensive research on Mg/graphene derivative composites as hydrogen storage materials, the fundamental understanding of the interaction with graphene derivatives and the morphological evolution of Mg remains to be elucidated, which ultimately leads to the capability of tuning their hydrogen storage properties. Here, we reveal the role of graphene defects in the size and morphology of Mg and demonstrate control of Mg homogeneity, by employing graphene oxides (GO) with different degrees of reduction. Mg nanocrystals confined by more reduced graphene oxides (rGO) not only show an increased activation energy barrier for de/absorption, but also a prolonged induction time compared to those on defective GO. Ab initio calculations rationalize that the interaction between Mg and GO defects dominates the Mg chemical potential, suppressing the growth of small Mg nanocrystals.


 Please wait while we load your content...
Please wait while we load your content...