Vanadium oxide integrated on hierarchically nanoporous copper for efficient electroreduction of CO2 to ethanol†
Abstract
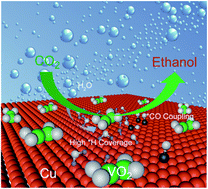
The electrochemical reduction of CO2 to an ethanol product is regarded as a highly promising route for CO2 utilization. However, the poor selectivity is still a critical challenge for increasing the yield of the specific ethanol. As a CO2 reduction catalyst, the hierarchically nanoporous copper integrated with vanadium oxide can achieve a 30.1% faradaic efficiency for CO2-to-ethanol production and an ethanol partial current density of −16 mA cm−2 at −0.62 V vs. RHE, corresponding to a 4-fold increase in activity compared to bare nanoporous Cu. It even delivers an ethanol partial current density that exceeds −39 mA cm−2 at −0.8 V vs. RHE in a flow-cell reactor. The hierarchically nanoporous Cu skeleton not only facilitates both electron and electrolyte transport but also provides a large specific surface area for high active site density. Density functional theory reveals that the vanadium oxide decorated Cu surface can facilitate water dissociation and optimize the hydrogen adsorption energy on Cu, lowering the energy barrier for the protonation of carbon dioxide and C–C coupling. Meanwhile, it can increase hydrogen proton coverage on the catalyst surface and inhibit dehydration, which are beneficial for breaking the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the *HCCOH intermediate, thus enhancing the faradaic efficiency of ethanol significantly. The highly efficient conversion of CO2 to ethanol demonstrates that the hybrid electrocatalyst is considered as a promising candidate for practical electrocatalytic CO2RR applications.
C bond of the *HCCOH intermediate, thus enhancing the faradaic efficiency of ethanol significantly. The highly efficient conversion of CO2 to ethanol demonstrates that the hybrid electrocatalyst is considered as a promising candidate for practical electrocatalytic CO2RR applications.


 Please wait while we load your content...
Please wait while we load your content...