Processing of fast-gelling hydrogel precursors in microfluidics by electrocoalescence of reactive species†
Abstract
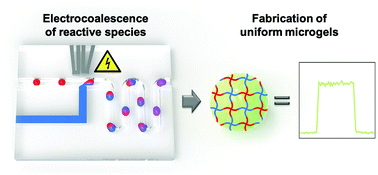
Microscopic hydrogels, also referred to as microgels, find broad application in life and materials science. A well-established technique for fabricating uniform microgels is droplet microfluidics. Here, optimal mixing of hydrogel precursor components is crucial to yield homogeneous microgels with respect to their morphology, mechanics, and distribution of functional moieties. However, when processing premixed polymer precursors that are highly reactive, fast or even instantaneous gelation inside fluid reservoirs or the microchannels of the flow cell commonly occurs, leading to an increase of fluid viscosity over time, and thus exacerbating the intrinsic control over fluid flow rates, droplet and microgel uniformity, which are key selling points of microfluidics in material design. To address these challenges, we utilize microflow cells with integrated electrodes, which enable fast addition and mixing of hydrogel precursors on demand by means of emulsion droplet coalescence. Here, two populations of surfactant-stabilized aqueous droplets – the first containing the material basis of the microgel, and the second containing another gel-forming component (e.g., a crosslinker) are formed at two consecutive microchannel junctions and merged via temporary thin-film instability. Our approach provides the ability to process such hydrogel systems that are otherwise challenging to process into uniform droplets and microgels by conventional droplet microfluidics. To demonstrate its versatility, we fabricate microgels with uniform shape and composition using fast hydrogelation via thiol-Michael addition reaction or non-covalent self-assembly. Furthermore, we elucidate the limitations of electrocoalescence of reactive hydrogel precursors by processing sodium alginate, crosslinked by calcium-induced ionic interactions. For this instantaneous type of hydrogelation, electrocoalescence of alginate and calcium ions does not result in the formation of morphologically isotropic microgels. Instead, it enables the creation of anisotropic microgel morphologies with tunable shape, which have previously only been achieved by selective crosslinking of elaborate higher-order emulsions or by aqueous two-phase systems as microgel templates.


 Please wait while we load your content...
Please wait while we load your content...