Actin filament alignment causes mechanical hysteresis in cross-linked networks†
Abstract
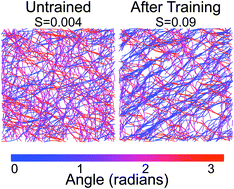
Cells dynamically control their material properties through remodeling of the actin cytoskeleton, an assembly of cross-linked networks and bundles formed from the biopolymer actin. We recently found that cross-linked networks of actin filaments reconstituted in vitro can exhibit adaptive behavior and thus serve as a model system to understand the underlying mechanisms of mechanical adaptation of the cytoskeleton. In these networks, training, in the form of applied shear stress, can induce asymmetry in the nonlinear elasticity. Here, we explore control over this mechanical hysteresis by tuning the concentration and mechanical properties of cross-linking proteins in both experimental and simulated networks. We find that this effect depends on two conditions: the initial network must exhibit nonlinear strain stiffening, and filaments in the network must be able to reorient during training. Hysteresis depends strongly and non-monotonically on cross-linker concentration, with a peak at moderate concentrations. In contrast, at low concentrations, where the network does not strain stiffen, or at high concentrations, where filaments are less able to rearrange, there is little response to training. Additionally, we investigate the effect of changing cross-linker properties and find that longer or more flexible cross-linkers enhance hysteresis. Remarkably plotting hysteresis against alignment after training yields a single curve regardless of the physical properties or concentration of the cross-linkers.
- This article is part of the themed collection: Soft Matter Most Popular 2021


 Please wait while we load your content...
Please wait while we load your content...
