Implementing Zn2+ ion and pH-value control into artificial mussel glue proteins by abstracting a His-rich domain from preCollagen†
Abstract
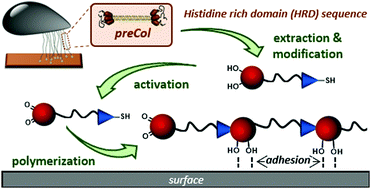
A His-rich domain of preCollagen-D found in byssal threads is derivatized with Cys and Dopa flanks to allow for mussel-inspired polymerization. Artificial mussel glue proteins are accessed that combine cysteinyldopa for adhesion with sequences for pH or Zn2+ induced β-sheet formation. The artificial constructs show strong adsorption to Al2O3, the resulting coatings tolerate hypersaline conditions and cohesion is improved by activating the β-sheet formation, that enhances E-modulus up to 60%.


 Please wait while we load your content...
Please wait while we load your content...