Revealing the contribution of singlet oxygen in the photoelectrochemical oxidation of benzyl alcohol†
Abstract
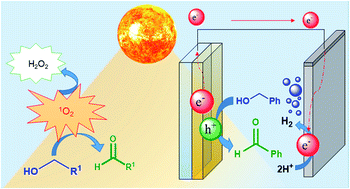
Photoelectrochemical approaches are finding use in the transformation of organic molecules beyond water oxidation and CO2 reduction. Among the different reactions that can be performed, the selective oxidation of alcohols to aldehydes is a transformation of great interest, as aldehydes are the starting materials for the preparation of more complex organic molecules with high added value. In order to develop efficient photoelectrochemical methodologies for this reaction, it is crucial to reveal all the different parameters that are involved in such light-driven processes. Herein we analyse in detail the effect of the light wavelength and atmosphere on the photoelectrochemical oxidation of benzyl alcohol to benzaldehyde using BiVO4 electrodes. Our studies demonstrate that an important contribution to the oxidation of alcohol is due to UV light-induced production of singlet oxygen, which is also responsible for the formation of hydrogen peroxide in the reaction media. These findings are key for identifying and evaluating the underlying mechanism involved in this type of photoelectrochemically induced oxidation, in order to avoid misinterpretations of the efficiencies.


 Please wait while we load your content...
Please wait while we load your content...