New insights into the disulfide bond formation enzymes in epidithiodiketopiperazine alkaloids†
Abstract
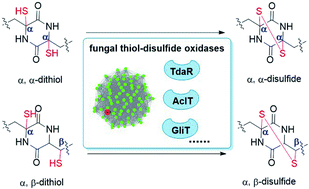
Epidithiodiketopiperazines (ETPs) are a group of bioactive fungal natural products and structurally feature unique transannular disulfide bridges between α, α or α, β carbons. However, no enzyme has yet been demonstrated to catalyse α, β-disulfide bond formation in these molecules. Through genome mining and gene deletion approaches in Trichoderma hypoxylon, we identified a putative biosynthetic gene cluster of pretrichodermamide A (1), which requires a FAD-dependent oxidoreductase, TdaR, for the irregular α, β-disulfide formation in 1 biosynthesis. In vitro assays of TdaR, together with AclT involved in aspirochlorine and GliT involved in gliotoxin biosynthesis, proved that all three enzymes catalyse not only the conversion of red-pretrichodermamide A (4) to α, β-disulfide-containing 1 but also that of red-gliotoxin (5) to α, α-disulfide-containing gliotoxin (6). These results provide new insights into the thiol-disulfide oxidases responsible for the disulfide bond formation in natural products with significant substrate and catalytic promiscuities.


 Please wait while we load your content...
Please wait while we load your content...