Migratory insertion of isocyanide into a ketenyl–tungsten bond as key step in cyclization reactions†
Abstract
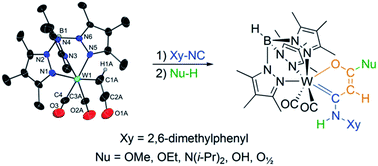
Treatment of the side-on tungsten alkyne complex of ethinylethyl ether [Tp*W(CO)2(η2-C,C′-HCCOCH2CH3)]+ {Tp* = hydridotris(3,4,5-trimethylpyrazolyl)borate} (2a) with n-Bu4NI afforded the end-on ketenyl complex [Tp*W(CO)2(κ1-HCCO)] (4a). This formal 16 ve complex bearing the prototype of a ketenyl ligand is surprisingly stable and converts only under activation by UV light or heat to form a dinuclear complex [Tp*2W2(CO)4(μ-CCH2)] (6). The ketenyl ligand in complex 4a underwent a metal template controlled cyclization reaction upon addition of isocyanides. The oxametallacycles [Tp*W(CO)2{κ2-C,O-C(NHXy)C(H)C(Nu)O}] {Nu = OMe (7), OEt (8), N(i-Pr)2 (9), OH (10), O1/2 (11)} were formed by coordination of Xy-NC (Xy = 2,6-dimethylphenyl) at 4a and subsequent migratory insertion (MI) into the W-ketenyl bond. The resulting intermediate is susceptible to addition reactions with protic nucleophiles. Compounds 2a-PF6, 4a/b, and 7–11 were fully characterized including XRD analysis. The cyclization mechanism has been confirmed both experimentally and by DFT calculations. In cyclic voltammetry, complexes 7–9 are characterized by a reversible W(II)/W(III) redox process. The dinuclear complex 11 however shows two separated redox events. Based on cyclic voltammetry measurements with different conducting electrolytes and IR spectroelectrochemical (SEC) measurements the W(II)/W(III) mixed valent complex 11+ is assigned to class II in terms of the Robin-Day classification.


 Please wait while we load your content...
Please wait while we load your content...