Dibenzocycloheptanones construction through a removable P-centered radical: synthesis of allocolchicine analogues†
Abstract
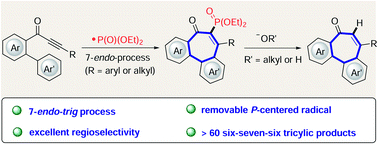
Dibenzocycloheptanones containing a tricyclic 6–7–6-system are present in numerous biologically active natural molecules. However, the simple and efficient preparation of derivatives containing a dibenzocycloheptanone scaffold remains difficult to date. Herein, we report a versatile strategy for the construction of these challenging seven-membered rings using a 7-endo-trig cyclization which is initiated by a phosphorus-centered radical. This approach provides a step-economical regime for the facile assembly of a wide range of phosphorylated dibenzocycloheptanones. Remarkably, we also have devised a traceless addition/exchange strategy for the preparation of dephosphorylated products at room temperature with excellent yields. Therefore, this protocol allows for the concise synthesis of biorelevant allocochicine derivatives.


 Please wait while we load your content...
Please wait while we load your content...