Multicomponent formation route to a new class of oxygen-based 1,3-dipoles and the modular synthesis of furans†
Abstract
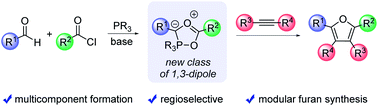
A new class of phosphorus-containing 1,3-dipoles can be generated by the multicomponent reaction of aldehydes, acid chlorides and the phosphonite PhP(catechyl). These 1,3-dipoles are formally cyclic tautomers of simple Wittig-type ylides, where the angle strain and moderate nucleophilicity in the catechyl-phosphonite favor their cyclization and also direct 1,3-dipolar cycloaddition to afford single regioisomers of substituted products. Coupling the generation of the dipoles with 1,3-dipolar cycloaddition offers a unique, modular route to furans from combinations of available aldehydes, acid chlorides and alkynes with independent control of all four substituents.


 Please wait while we load your content...
Please wait while we load your content...