C–H functionalisation tolerant to polar groups could transform fragment-based drug discovery (FBDD)†
Abstract
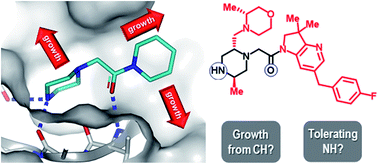
We have analysed 131 fragment-to-lead (F2L) examples targeting a wide variety of protein families published by academic and industrial laboratories between 2015–2019. Our assessment of X-ray structural data identifies the most common polar functional groups involved in fragment-protein binding are: N–H (hydrogen bond donors on aromatic and aliphatic N–H, amides and anilines; totalling 35%), aromatic nitrogen atoms (hydrogen bond acceptors; totalling 23%), and carbonyl oxygen group atoms (hydrogen bond acceptors on amides, ureas and ketones; totalling 22%). Furthermore, the elaboration of each fragment into its corresponding lead is analysed to identify the nominal synthetic growth vectors. In ∼80% of cases, growth originates from an aromatic or aliphatic carbon on the fragment and more than 50% of the total bonds formed are carbon–carbon bonds. This analysis reveals that growth from carbocentric vectors is key and therefore robust C–H functionalisation methods that tolerate the innate polar functionality on fragments could transform fragment-based drug discovery (FBDD). As a further resource to the community, we have provided the full data of our analysis as well as an online overlay page of the X-ray structures of the fragment hit and leads: https://astx.com/interactive/F2L-2021/
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...