Solvent-controlled photocatalytic divergent cyclization of alkynyl aldehydes: access to cyclopentenones and dihydropyranols†‡
Abstract
Divergent synthesis is a powerful strategy for the fast assembly of different molecular scaffolds from identical starting materials. We describe here a solvent-controlled photocatalytic divergent cyclization of alkynyl aldehydes with sulfonyl chlorides for the direct construction of highly functionalized cyclopentenones and dihydropyranols that widely exist in bioactive molecules and natural products. Density functional theory calculations suggest that a unique N,N-dimethylacetamide-assisted 1,2-hydrogen transfer of alkoxy radicals is responsible for the cyclopentenone formation, whereas a C–C cleavage accounts for the selective production of dihydropyranols in acetonitrile and water at 50 °C. Given the simple and mild reaction conditions, excellent functional group compatibility, forming up to four chemical bonds, and tunable selectivity, it may find wide applications in synthetic chemistry.
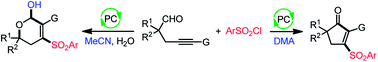
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...