Acid-catalysed liquid-to-solid transitioning of arylazoisoxazole photoswitches†
Abstract
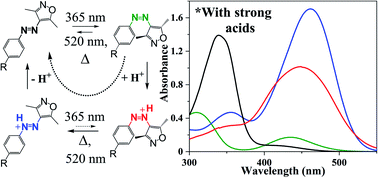
Molecular photoswitches play a vital role in the development of responsive materials. These molecular building blocks are particularly attractive when multiple stimuli can be combined to bring about physical changes, sometimes leading to unexpected properties and functions. The arylazoisoxazole molecular switch was recently shown to be capable of efficient photoreversible solid-to-liquid phase transitions with application in photoswitchable surface adhesion. Here, we show that the arylazoisoxazole forms thermally stable and photoisomerisable protonated Z- and E-isomers in an apolar aprotic solvent when the pKa of the applied acid is sufficiently low. The tuning of isomerisation kinetics from days to seconds by the pKa of the acid not only opens up new reactivity in solution, but also the solid-state photoswitching of azoisoxazoles can be efficiently reversed with selected acid vapours, enabling acid-gated photoswitchable surface adhesion.


 Please wait while we load your content...
Please wait while we load your content...