On 1,3-phosphaazaallenes and their diverse reactivity†
Abstract
1,3-Phosphaazaallenes are heteroallenes of the type RP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
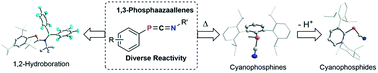
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NR′ and little is known about their reactivity. In here we describe the straightforward synthesis of ArPCNR (Ar = Mes*, 2,4,6-tBu-C6H2; MesTer, 2.6-(2,4,6-Me3C6H2)–C6H3; DipTer, 2.6-(2,6-iPr2C6H2)–C6H3; R = tBu; Xyl, 2,6-Me2C6H3) starting from phospha-Wittig reagents ArPPMe3 and isonitriles CNR. It is further shown that ArPCNtBu are thermally labile with respect to the loss of iso-butene and it is shown that the cyanophosphines ArP(H)CN are synthetically feasible and form the corresponding phosphanitrilium borates with B(C6F5)3, whereas deprotonation of DipTerP(H)CN was shown to give an isolable cyanidophosphide. Lastly, the reactivity of ArPCNR towards Pier's borane was investigated, showing hydroboration of the C
NR′ and little is known about their reactivity. In here we describe the straightforward synthesis of ArPCNR (Ar = Mes*, 2,4,6-tBu-C6H2; MesTer, 2.6-(2,4,6-Me3C6H2)–C6H3; DipTer, 2.6-(2,6-iPr2C6H2)–C6H3; R = tBu; Xyl, 2,6-Me2C6H3) starting from phospha-Wittig reagents ArPPMe3 and isonitriles CNR. It is further shown that ArPCNtBu are thermally labile with respect to the loss of iso-butene and it is shown that the cyanophosphines ArP(H)CN are synthetically feasible and form the corresponding phosphanitrilium borates with B(C6F5)3, whereas deprotonation of DipTerP(H)CN was shown to give an isolable cyanidophosphide. Lastly, the reactivity of ArPCNR towards Pier's borane was investigated, showing hydroboration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in Mes*PCNtBu to give a hetero-butadiene, while with DipTerPCNXyl the formation of the Lewis acid–base adduct with a B–P linkage was observed.
N bond in Mes*PCNtBu to give a hetero-butadiene, while with DipTerPCNXyl the formation of the Lewis acid–base adduct with a B–P linkage was observed.


 Please wait while we load your content...
Please wait while we load your content...