Nickel-mediated N–N bond formation and N2O liberation via nitrogen oxyanion reduction†
Abstract
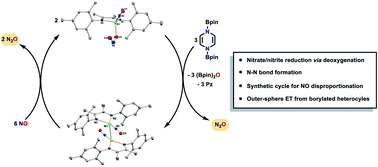
The syntheses of (DIM)Ni(NO3)2 and (DIM)Ni(NO2)2, where DIM is a 1,4-diazadiene bidentate donor, are reported to enable testing of bis boryl reduced N-heterocycles for their ability to carry out stepwise deoxygenation of coordinated nitrate and nitrite, forming O(Bpin)2. Single deoxygenation of (DIM)Ni(NO2)2 yields the tetrahedral complex (DIM)Ni(NO)(ONO), with a linear nitrosyl and κ1-ONO. Further deoxygenation of (DIM)Ni(NO)(ONO) results in the formation of dimeric [(DIM)Ni(NO)]2, where the dimer is linked through a Ni–Ni bond. The lost reduced nitrogen byproduct is shown to be N2O, indicating N–N bond formation in the course of the reaction. Isotopic labelling studies establish that the N–N bond of N2O is formed in a bimetallic Ni2 intermediate and that the two nitrogen atoms of (DIM)Ni(NO)(ONO) become symmetry equivalent prior to N–N bond formation. The [(DIM)Ni(NO)]2 dimer is susceptible to oxidation by AgX (X = NO3−, NO2−, and OTf−) as well as nitric oxide, the latter of which undergoes nitric oxide disproportionation to yield N2O and (DIM)Ni(NO)(ONO). We show that the first step in the deoxygenation of (DIM)Ni(NO)(ONO) to liberate N2O is outer sphere electron transfer, providing insight into the organic reductants employed for deoxygenation. Lastly, we show that at elevated temperatures, deoxygenation is accompanied by loss of DIM to form either pyrazine or bipyridine bridged polymers, with retention of a BpinO− bridging ligand.


 Please wait while we load your content...
Please wait while we load your content...