Chemical synthesis of linear ADP-ribose oligomers up to pentamer and their binding to the oncogenic helicase ALC1†
Abstract
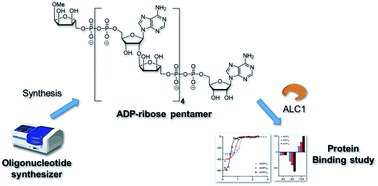
ADP-ribosylation is a pivotal post-translational modification that mediates various important cellular processes producing negatively charged biopolymer, poly (ADP-ribose), the functions of which need further elucidation. Toward this end, the availability of well-defined ADP-ribose (ADPr) oligomers in sufficient quantities is a necessity. In this work, we demonstrate the chemical synthesis of linear ADPr oligomers of defined, increasing length using a modified solid phase synthesis method. An advanced phosphoramidite building block temporarily protected with the base sensitive Fm-group was designed and implemented in the repeating pyrophosphate formation via a P(V)–P(III) coupling procedure on Tentagel solid support. Linear ADPr oligomers up to a pentamer were successfully synthesized and their affinity for the poly-(ADP-ribose)-binding macrodomain of the human oncogenic helicase and chromatin remodeling enzyme ALC1 was determined. Our data reveal a length-dependent binding manner of the nucleic acid, with larger ADPr oligomers exhibiting higher binding enthalpies for ALC1, illustrating how the activity of this molecular machine is gated by PAR.


 Please wait while we load your content...
Please wait while we load your content...