Sulfated poly-amido-saccharides (sulPASs) are anticoagulants in vitro and in vivo†
Abstract
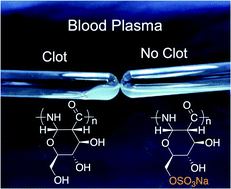
Anticoagulant therapeutics are a mainstay of modern surgery and of clotting disorder management such as venous thrombosis, yet performance and supply limitations exist for the most widely used agent – heparin. Herein we report the first synthesis, characterization, and performance of sulfated poly-amido-saccharides (sulPASs) as heparin mimetics. sulPASs inhibit the intrinsic pathway of coagulation, specifically FXa and FXIa, as revealed by ex vivo human plasma clotting assays and serine protease inhibition assays. sulPASs activity positively correlates with molecular weight and degree of sulfation. Importantly, sulPASs are not degraded by heparanases and are non-hemolytic. In addition, their activity is reversed by protamine sulfate, unlike small molecule anticoagulants. In an in vivo murine model, sulPASs extend clotting time in a dose dependent manner with bleeding risk comparable to heparin. These findings support continued development of synthetic anticoagulants to address the clinical risks and shortages associated with heparin.


 Please wait while we load your content...
Please wait while we load your content...