Electrostatic regulation of blue copper sites†
Abstract
In the last 50 years, the blue copper proteins became central targets of investigation. Extensive experiments focused on the Cu coordination to probe the effect of local perturbations on its properties. We found that local electric fields, generated by charged residues evolutionarily placed throughout the protein edifice, mainly second sphere, but also more remotely, constitute an additional significant factor regulating blue copper proteins. These fields are not random, but exhibit a highly specific directionality, negative with respect to the 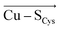 and
and 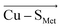 vectors in the Cu first shell. The field magnitude contributes to fine-tuning of the geometric and electronic properties of Cu sites in individual blue copper proteins. Specifically, the local electric fields evidently control the Cu–SMet bond distance, Cu(II)–SCys bond covalency, and the energies of the frontier molecular orbitals, which, in turn, govern the Cu(II/I) reduction potential and the relative absorption intensities at 450 nm and 600 nm.
vectors in the Cu first shell. The field magnitude contributes to fine-tuning of the geometric and electronic properties of Cu sites in individual blue copper proteins. Specifically, the local electric fields evidently control the Cu–SMet bond distance, Cu(II)–SCys bond covalency, and the energies of the frontier molecular orbitals, which, in turn, govern the Cu(II/I) reduction potential and the relative absorption intensities at 450 nm and 600 nm.



 Please wait while we load your content...
Please wait while we load your content...