The mechanism behind enhanced reactivity of unsaturated phosphorus(v) electrophiles towards thiols†‡
Abstract
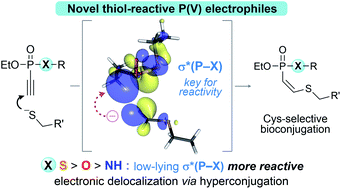
Vinyl- and ethynyl phosphorus(V) electrophiles are a versatile class of thiol-reactive reagents suitable for cysteine-selective peptide and protein modifications, especially for the generation of antibody conjugates. Herein we investigated the reactivity of various P(V) reagents towards thiol addition. Complementing previous studies, we observed that the heteroatoms X (X = S, O, NH) as well as the vinyl- vs. ethynyl-substituent bound to phosphorus greatly influence the overall reactivity. These experimentally observed trends, as well as the high Z-selectivity for thiol additions to ethynyl derivatives, were further elucidated using DFT calculations. Hyperconjugation was a key means of stabilizing the intermediate generated upon the thiol addition, thus determining both the reactivity and stereoselectivity of unsaturated P(V) electrophiles. Specifically, the energetically low-lying σ antibonding orbital of the P–S bond more readily stabilizes the electron density from the lone pair (LP) of the generated carbanion, rendering the phosphonothiolates more reactive compared to the derivatives bearing oxygen and nitrogen. Our studies provide a detailed mechanistic picture for designing P(V)-based electrophiles with fine-tuned reactivity profiles.


 Please wait while we load your content...
Please wait while we load your content...