Stereoselective β-mannosylations and β-rhamnosylations from glycosyl hemiacetals mediated by lithium iodide†
Abstract
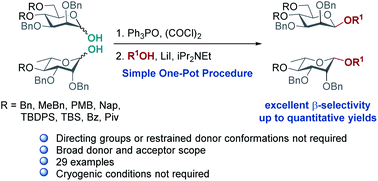
Stereoselective β-mannosylation is one of the most challenging problems in the synthesis of oligosaccharides. Herein, a highly selective synthesis of β-mannosides and β-rhamnosides from glycosyl hemi-acetals is reported, following a one-pot chlorination, iodination, glycosylation sequence employing cheap oxalyl chloride, phosphine oxide and LiI. The present protocol works excellently with a wide range of glycosyl acceptors and with armed glycosyl donors. The method doesn't require conformationally restricted donors or directing groups; it is proposed that the high β-selectivities observed are achieved via an SN2-type reaction of α-glycosyl iodide promoted by lithium iodide.


 Please wait while we load your content...
Please wait while we load your content...