Folding-controlled assembly of ortho-phenylene-based macrocycles†
Abstract
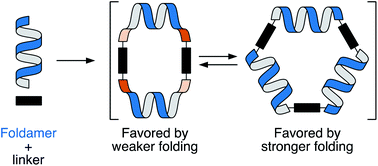
The self-assembly of foldamers into macrocycles is a simple approach to non-biological higher-order structure. Previous work on the co-assembly of ortho-phenylene foldamers with rod-shaped linkers has shown that folding and self-assembly affect each other; that is, the combination leads to new emergent behavior, such as access to otherwise unfavorable folding states. To this point this relationship has been passive. Here, we demonstrate control of self-assembly by manipulating the foldamers' conformational energy surfaces. A series of o-phenylene decamers and octamers have been assembled into macrocycles using imine condensation. Product distributions were analyzed by gel-permeation chromatography and molecular geometries extracted from a combination of NMR spectroscopy and computational chemistry. The assembly of o-phenylene decamers functionalized with alkoxy groups or hydrogens gives both [2 + 2] and [3 + 3] macrocycles. The mixture results from a subtle balance of entropic and enthalpic effects in these systems: the smaller [2 + 2] macrocycles are entropically favored but require the oligomer to misfold, whereas a perfectly folded decamer fits well within the larger [3 + 3] macrocycle that is entropically disfavored. Changing the substituents to fluoro groups, however, shifts assembly quantitatively to the [3 + 3] macrocycle products, even though the structural changes are well-removed from the functional groups directly participating in bond formation. The electron-withdrawing groups favor folding in these systems by strengthening arene–arene stacking interactions, increasing the enthalpic penalty to misfolding. The architectural changes are substantial even though the chemical perturbation is small: analogous o-phenylene octamers do not fit within macrocycles when perfectly folded, and quantitatively misfold to give small macrocycles regardless of substitution. Taken together, these results represent both a high level of structural control in structurally complex foldamer systems and the demonstration of large-amplitude structural changes as a consequence of a small structural effects.


 Please wait while we load your content...
Please wait while we load your content...