Selective aldehyde reductions in neutral water catalysed by encapsulation in a supramolecular cage†
Abstract
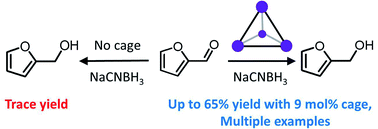
The enhancement of reactivity inside supramolecular coordination cages has many analogies to the mode of action of enzymes, and continues to inspire the design of new catalysts for a range of reactions. However, despite being a near-ubiquitous class of reactions in organic chemistry, enhancement of the reduction of carbonyls to their corresponding alcohols remains very much underexplored in supramolecular coordination cages. Herein, we show that encapsulation of small aromatic aldehydes inside a supramolecular coordination cage allows the reduction of these aldehydes with the mild reducing agent sodium cyanoborohydride to proceed with high selectivity (ketones and esters are not reduced) and in good yields. In the absence of the cage, low pH conditions are essential for any appreciable conversion of the aldehydes to the alcohols. In contrast, the specific microenvironment inside the cage allows this reaction to proceed in bulk solution that is pH-neutral, or even basic. We propose that the cage acts to stabilise the protonated oxocarbenium ion reaction intermediates (enhancing aldehyde reactivity) whilst simultaneously favouring the encapsulation and reduction of smaller aldehydes (which fit more easily inside the cage). Such dual action (enhancement of reactivity and size-selectivity) is reminiscent of the mode of operation of natural enzymes and highlights the tremendous promise of cage architectures as selective catalysts.
- This article is part of the themed collection: Most popular 2021 supramolecular chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...