Stereoselective synthesis of unnatural α-amino acid derivatives through photoredox catalysis†
Abstract
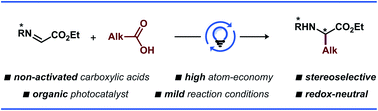
A protocol for stereoselective C-radical addition to a chiral glyoxylate-derived N-sulfinyl imine was developed through visible light-promoted photoredox catalysis, providing a convenient method for the synthesis of unnatural α-amino acids. The developed protocol allows the use of ubiquitous carboxylic acids as radical precursors without prior derivatization. The protocol utilizes near-stoichiometric amounts of the imine and the acid radical precursor in combination with a catalytic amount of an organic acridinium-based photocatalyst. Alternative mechanisms for the developed transformation are discussed and corroborated by experimental and computational studies.


 Please wait while we load your content...
Please wait while we load your content...