N-Oxide S–O chalcogen bonding in conjugated materials†
Abstract
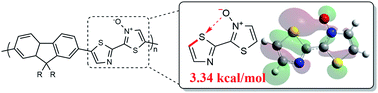
Non-covalent bonding interactions, such as chalcogen bonding, can have a substantial effect on the electronic and physical properties of conjugated polymers and is largely dependent on the strength of interaction. Functional groups that are traditionally used to instill chalcogen bonding such as alkoxy or fluorine substituents can demand challenging synthetic effort, as well as have drastic effects on the electronics of a π-system. The incorporation of a N-oxide functionality into bithiazole-containing materials, a synthetically simple transformation, has been entirely overlooked until now. A systematic analysis of the effects of N-oxidation on the electronic and physical properties of bithiazole-containing materials has been undertaken. N-Oxidation has been found to affect the electronic band gap through increase of the HOMO and lowering of the LUMO. Furthermore, exceptionally strong intramolecular S–O chalcogen bonding interactions in the bithiazole core contribute to rigidification of the conjugated system. Computational analysis of this system has shown this N-oxide chalcogen bonding interaction to be significantly stronger than other chalcogen bonding interactions commonly exploited in conjugated materials.


 Please wait while we load your content...
Please wait while we load your content...