Abstract
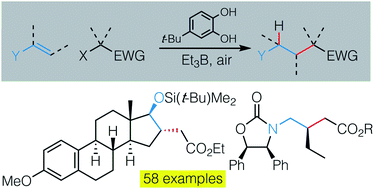
A general method for the hydroalkylation of electron-rich terminal and non-terminal alkenes such as enol esters, alkenyl sulfides, enol ethers, silyl enol ethers, enamides and enecarbamates has been developed. The reactions are carried out at room temperature under air initiation in the presence of triethylborane acting as a chain transfer reagent and 4-tert-butylcatechol (TBC) as a source of hydrogen atom. The efficacy of the reaction is best explained by very favorable polar effects supporting the chain process and minimizing undesired polar reactions. The stereoselective hydroalkylation of chiral N-(alk-1-en-1-yl)oxazolidin-2-ones takes place with good to excellent diastereocontrol.


 Please wait while we load your content...
Please wait while we load your content...