Trioxatriangulenium (TOTA+) as a robust carbon-based Lewis acid in frustrated Lewis pair chemistry†
Abstract
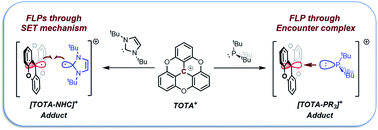
We report the reactivity between the water stable Lewis acidic trioxatriangulenium ion (TOTA+) and a series of Lewis bases such as phosphines and N-heterocyclic carbene (NHC). The nature of the Lewis acid–base interaction was analyzed via variable temperature (VT) NMR spectroscopy, single-crystal X-ray diffraction, UV-visible spectroscopy, and DFT calculations. While small and strongly nucleophilic phosphines, such as PMe3, led to the formation of a Lewis acid–base adduct, frustrated Lewis pairs (FLPs) were observed for sterically hindered bases such as P(tBu)3. The TOTA+–P(tBu)3 FLP was characterized as an encounter complex, and found to promote the heterolytic cleavage of disulfide bonds, formaldehyde fixation, dehydrogenation of 1,4-cyclohexadiene, heterolytic cleavage of the C–Br bonds, and interception of Staudinger reaction intermediates. Moreover, TOTA+ and NHC were found to first undergo single-electron transfer (SET) to form [TOTA]·[NHC]˙+, which was confirmed via electron paramagnetic resonance (EPR) spectroscopy, and subsequently form a [TOTA–NHC]+ adduct or a mixture of products depending the reaction conditions used.
- This article is part of the themed collection: Most popular 2021 main group, inorganic and organometallic chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...