Organophosphorus-catalyzed relay oxidation of H-Bpin: electrophilic C–H borylation of heteroarenes†
Abstract
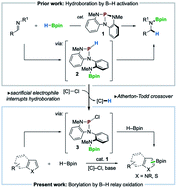
A nontrigonal phosphorus triamide (1, P{N[o-NMe-C6H4]2}) is shown to catalyze C–H borylation of electron-rich heteroarenes with pinacolborane (HBpin) in the presence of a mild chloroalkane reagent. C–H borylation proceeds for a range of electron-rich heterocycles including pyrroles, indoles, and thiophenes of varied substitution. Mechanistic studies implicate an initial P–N cooperative activation of HBpin by 1 to give P-hydrido diazaphospholene 2, which is diverted by Atherton–Todd oxidation with chloroalkane to generate P-chloro diazaphospholene 3. DFT calculations suggest subsequent oxidation of pinacolborane by 3 generates chloropinacolborane (ClBpin) as a transient electrophilic borylating species, consistent with observed substituent effects and regiochemical outcomes. These results illustrate the targeted diversion of established reaction pathways in organophosphorus catalysis to enable a new mode of main group-catalyzed C–H borylation.


 Please wait while we load your content...
Please wait while we load your content...