The site pair matching of a tandem Au/CuO–CuO nanocatalyst for promoting the selective electrolysis of CO2 to C2 products†
Abstract
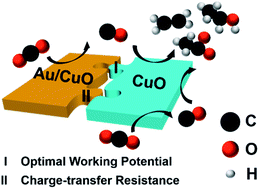
Tandem catalysis, in which a CO2-to-C2 process is divided into a CO2-to-CO/*CO step and a CO/*CO-to-C2 step, is promising for enhancing the C2 product selectivity when using Cu-based electrochemical CO2 reduction catalysts. In this work, a nanoporous hollow Au/CuO–CuO tandem catalyst was used for catalyzing the eCO2RR, which exhibited a C2 product FE of 52.8% at −1.0 V vs. RHE and a C2 product partial current density of 78.77 mA cm−2 at −1.5 V vs. RHE. In addition, the C2 product FE stably remained at over 40% over a wide potential range, from −1.0 V to −1.5 V. This superior performance was attributed to good matching in terms of the optimal working potential and charge-transfer resistance between CO/*CO-production sites (Au/CuO) and CO/*CO-reduction sites (CuO). This site pair matching effect ensured sufficient supplies of CO/*CO and electrons at CuO sites at the working potentials, thus dramatically enhancing the formation rate of C2 products.


 Please wait while we load your content...
Please wait while we load your content...