A library of new bifunctional alkenes obtained by a highly regiodivergent silylation of 1,5-hexadiene†
Abstract
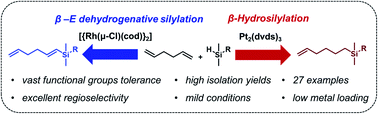
An efficient methodology for the synthesis of two groups of silicon-containing alkenes is reported. It includes a highly regioselective functionalization of 1,5-hexadiene through hydrosilylation and dehydrogenative silylation with organofunctional silanes and siloxanes. The established conditions enable selective monofunctionalization of 1,5-hexadiene regardless of the organosilicon modifier used as well as the type of functional group bonded to the silicon-based compound. All products were isolated and fully characterized by NMR spectroscopy and MS techniques.


 Please wait while we load your content...
Please wait while we load your content...