Spatial distribution of isobaric androgens in target tissues using chemical derivatization and MALDI-2 on a trapped ion mobility quadrupole time-of-flight instrument†
Abstract
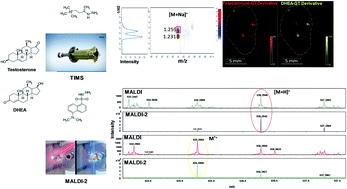
Prostate cancer is initially treated via androgen deprivation therapy (ADT), a highly successful treatment in the initial pursuit of tumour regression, but commonly restricted by the eventual emergence of a more lethal ‘castrate resistant’ (CRPC) form of the disease. Intracrine pathways that utilize dehydroepiandrosterone (DHEA) or other circulatory precursor steroids are thought to generate relevant levels of growth-stimulating androgens such as testosterone (T) and dihydrotestosterone (DHT). Decoding this tissue-specific metabolic pathway is key for the development of novel therapeutic treatments. Mass spectrometry imaging (MSI) is an analytical technique that allows the visualization of the distribution of numerous classes of biomolecules within tissue sections. The analysis of androgens by liquid chromatography mass spectrometry (LC/MS)-based methods however presents a challenge due to their generally poor ionization efficiency and low physiological endogenous levels. In MSI, on-tissue chemical derivatization (OTCD) has enabled the limits of steroids to be imaged within tissues to be pushed by overcoming poor ionization performance. However, isobaric interference of key androgen derivatives such as T and DHEA can severely hamper studying the intracrinology in several diseases. Here, we have evaluated the use of laser induced post-ionization (MALDI-2) combined with trapped ion mobility separation (TIMS) and orthogonal time-of-flight (QTOF) MS for the visualization of isobaric derivatized androgens in murine tumour xenograft at about 50 μm spatial resolution. With this combination, isobaric T and DHEA were separated in tissue sections and the signals of derivatized steroids enhanced by about 20 times. The combination of TIMS and MALDI-2 thus shows unique potential to study tissue intracrinology within target tissues. This could offer the opportunity for many novel insights into tissue-specific androgen biology.


 Please wait while we load your content...
Please wait while we load your content...