Preparative separation of 1,1-diphenyl-2-picrylhydrazyl inhibitors originating from Saxifraga sinomontana employing medium-pressure liquid chromatography in combination with reversed-phase liquid chromatography†
Abstract
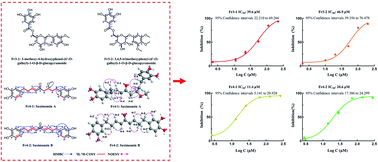
Traditional Tibetan medicines elaborately document the health benefits of Saxifraga sinomontana. However, there have been limited reports on its chemical make-up, presumably because of the complicated separation and purification process. In this work, a methanolic extract of Saxifraga sinomontana was utilized for targeted separation of 4 key 1,1-diphenyl-2-picrylhydrazyl inhibitors employing the medium-pressure liquid chromatography, reversed-phase liquid chromatography in combination with on-line reversed-phase liquid chromatography-1,1-diphenyl-2-picrylhydrazyl detection. Pre-treatment of the sample was carried out by employing medium-pressure liquid chromatography using MCI GEL® CHP20P as the stationary phase, furnishing 2.4 g of fraction Fr3 and 3.4 g of fraction Fr4 (the percentage retrieval was 32.7%). The 1,1-diphenyl-2-picrylhydrazyl inhibitors contained in fractions Fr3 and Fr4 were subjected to additional separation using a C18 (ReproSil-Pur C18 AQ) column and yielded 106.2 mg of Fr3-1, 246.9 mg of Fr3-2, 248.5 mg of Fr4-1 and 41.8 mg of Fr4-2. The degree of purity, structures and 1,1-diphenyl-2-picrylhydrazyl inhibition activity of the isolated DPPH inhibitors were determined, and four 1,1-diphenyl-2-picrylhydrazyl inhibitors including two new diarylnonanoids (3-methoxy-4-hydroxyphenol-(6′-O-galloyl)-1-O-β-D-glucopyrano side with IC50 of 39.6 μM, 3,4,5-trimethoxyphenyl-(6′-O-galloyl)-1-O-β-D-glucopyranoside with IC50 of 46.9 μM, saximonsin A with IC50 of 11.4 μM, and saximonsin B with IC50 of 20.6 μM) were isolated with a percentage purity above 95%. The methodology thus evolved has good efficacy for preparatively isolating high-purity 1,1-diphenyl-2-picrylhydrazyl inhibitors from extracts of Saxifraga sinomontana and could be efficiently utilized for rapidly isolating 1,1-diphenyl-2-picrylhydrazyl inhibitors from other natural products.


 Please wait while we load your content...
Please wait while we load your content...