Theoretical study of ternary silver fluorides AgMF4 (M = Cu, Ni, Co) formation at pressures up to 20 GPa†‡
Abstract
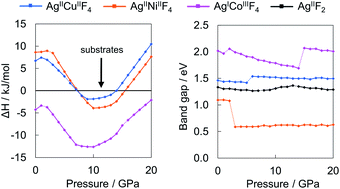
Only several compounds bearing the Ag(II) cation and other paramagnetic transition metal cations are known experimentally. Herein, we predict in silico stability and crystal structures of hypothetical ternary silver(II) fluorides with copper, nickel and cobalt in 1 : 1 stoichiometry at a pressure range from 0 GPa up to 20 GPa employing the evolutionary algorithm in combination with DFT calculations. The calculations show that AgCoF4 could be synthesized already at ambient conditions but this compound would host diamagnetic Ag(I) and high-spin Co(III). Although none of the compounds bearing Ag(II) could be preferred over binary substrates at ambient conditions, at increased pressure ternary fluorides of Ag(II) featuring Cu(II) and Ni(II) could be synthesized, in the pressure windows of 7–14 and 8–15 GPa, respectively. All title compounds would be semiconducting and demonstrate magnetic ordering. Compounds featuring Ni(II) and particularly Co(II) should exhibit fundamental band gaps much reduced with respect to pristine AgF2. The presence of Cu(II) and Ni(II) does not lead to electronic doping to AgF2 layers, while Co(II) tends to reduce Ag(II) entirely to Ag(I).


 Please wait while we load your content...
Please wait while we load your content...