Highly robust, novel aluminum counter cation-based monophosphate tungsten bronze electro-catalysts for oxygen evolution in acidic solution†
Abstract
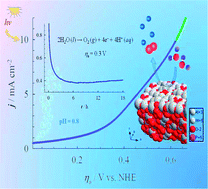
This study describes the successful synthesis of novel bronze with a low tungsten oxidation state for the efficient electro-catalytic oxidation of water. An extraordinarily robust monophosphate tungsten bronze (MPTB)-modified graphite anode was successfully fabricated for the oxygen evolution reaction (OER) at a thermodynamic potential of 1.23 V in H2SO4 acidic solution. Several Al, Cr and Fe counter-cation-based MPTBs were synthesized by the solution combustion method. Novel Al-based MPTBs calcined at 700 °C in O2 (AlO7) showed almost zero onset overpotential, high current density, high turnover frequency for OER and steady catalysis in repeated use even after 30 weeks. The orthorhombic AlO7 comprising crystallites of 9.89 nm and an indirect band gap (1.89 eV), is an unusually stable MPTB that contains 98% W5+ state stabilized with the Al3+ counter cation. The catalysis decreases as the ratio of W5+ : W6+ in MPTBs decreases and [410] and [601] facets play main roles in the first H2O association and nucleophilic attack of the second H2O molecule on the catalyst surface. Thus, MPTBs can be non-noble metal anode materials for robust acidic H2O electrolyzers.


 Please wait while we load your content...
Please wait while we load your content...