A more sustainable isothiocyanate synthesis by amine catalyzed sulfurization of isocyanides with elemental sulfur†
Abstract
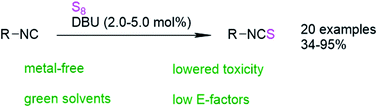
Isothiocyanates (ITCs) are typically prepared using amines and highly toxic reagents such as thiophosgene, its derivatives, or CS2. In this work, an investigation of a multicomponent reaction (MCR) using isocyanides, elemental sulfur and amines revealed that isocyanides can be converted to isothiocyanates using sulfur and catalytic amounts of amine bases, especially DBU (down to 2 mol%). This new catalytic reaction was optimized in terms of sustainability, especially considering benign solvents such as Cyrene™ or γ-butyrolactone (GBL) under moderate heating (40 °C). Purification by column chromatography was further optimized to generate less waste by maintaining high purity of the product. Thus, E-factors as low as 0.989 were achieved and the versatility of this straightforward procedure was shown by converting 20 different isocyanides under catalytic conditions, while obtaining moderate to high yields (34–95%).


 Please wait while we load your content...
Please wait while we load your content...