Thiol-yne click reaction: an interesting way to derive thiol-provided catechols†
Abstract
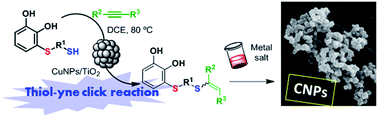
The hydrothiolation of activated alkynes is presented as an attractive and powerful way to functionalize thiols bearing catechols. The reaction was promoted by a heterogeneous catalyst composed of copper nanoparticles supported on TiO2 (CuNPs/TiO2) in 1,2-dichloroethane (1,2-DCE) under heating at 80 °C. The catalyst could be recovered and reused in three consecutive cycles, showing a slight decrease in its catalytic activity. Thiol derivatives bearing catechol moieties, obtained through a versatile Michael addition, were reacted with different activated alkynes, such as methyl propiolate, propiolic acid, propiolamide or 2-ethynylpyridine. The reaction was shown to be regio- and stereoselective towards anti-Markovnikov Z-vinyl sulfide in most cases studied. Finally, some catechol derivatives obtained were tested as ligands in the preparation of coordination polymer nanoparticles (CNPs), by taking the advantage of their different coordination sites with metals such as iron and cobalt.
- This article is part of the themed collection: A celebration of Latin American research in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...