Adsorption of CO2 on the ω-Fe (0001) surface: insights from density functional theory
Abstract
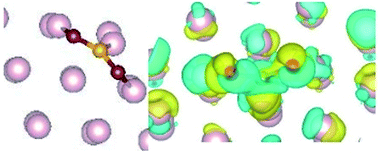
The stabilization of a hexagonal phase known as the ω-phase in steel has recently been identified. The presence of C in steel samples is found to be helping the formation of this otherwise meta stable phase. This indicates that the probability of degradation of the surface is high in steel samples containing the ω-phase, through surface adsorption. Here we calculate the adsorption process of CO2 on the ω-Fe(0001) surface, for different sites and find that it strongly adsorbs horizontally with a bent configuration. The adsorption is characterized by significant charge transfer from the surface Fe atoms to the CO2 molecule, and structural modification of the molecule is occurring. The density of states calculations indicate that hybridization and subsequent charge transfer is probable between the d orbitals of Fe and p orbitals of CO2, resulting in strong chemisorption, that further leads to spontaneous dissociation of the molecule.


 Please wait while we load your content...
Please wait while we load your content...