Regioselective cascade annulation of indoles with alkynediones for construction of functionalized tetrahydrocarbazoles triggered by Cp*RhIII-catalyzed C–H activation†
Abstract
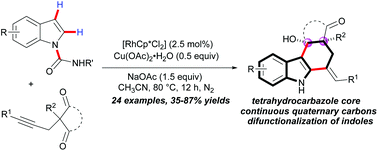
An efficient regioselective and stereoselective cascade annulation of indoles with alkynediones has been demonstrated via a Cp*RhIII-catalyzed indole C2–H activation by using N-carboamide directing groups in good to excellent yields with a broad substrate scope, which further realized the C2 and C3 difunctionalization of indoles in one-pot. This methodology simultaneously provides a novel and straightforward approach to free (NH) tetrahydrocarbazoles with continuous quaternary carbons and pyridoindolones, which are valuable intermediates in total synthesis of the related indole alkaloids.


 Please wait while we load your content...
Please wait while we load your content...