A convenient access to fluorophosphonium triflate salts by electrophilic fluorination and anion exchange†
Abstract
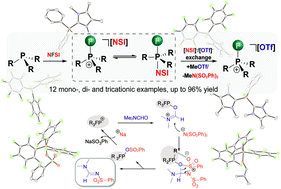
The in situ electrophilic fluorination of phosphanes with commercially available bench-stable N-fluorobenzenesulfonimide (NFSI), followed by subsequent methylation of the [N(PhSO2)2]− anion with MeOTf yields a family of electrophilic fluorophosphonium cations as triflate salts. Most of these fluorophosphonium triflate salts are remarkably Lewis acidic and form isolable adducts in stoichiometric reactions with suitable donors such as N,N-dimethylformamide (DMF). Furthermore, their catalytic capabilities were tested in the transformation of formamides into N-sulfonyl formamidines in the reaction with Na[N(SO2Ph)2] and the proposed mechanism is validated by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...