Different effects of metal-NHC bond cleavage on the Pd/NHC and Ni/NHC catalyzed α-arylation of ketones with aryl halides†
Abstract
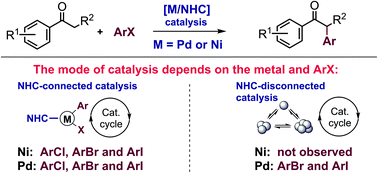
Recently, the dynamic nature of the metal-NHC bond has been proposed and the key role of chemical evolution in changing the nature of catalytically active sites is now an emerging topic. A comparative analysis of the ketone α-arylation reaction with aryl halides, catalyzed by M/NHC complexes, was carried out in the present study and showed a fundamental difference in the behavior of the catalytic system for M = Ni and Pd. In situ evolution of Ni/NHC complexes with cleavage of the Ni-NHC bond leads to complete deactivation of catalytic systems, regardless of the nature of the aryl halide ArX (X = Cl, Br, I). However, upon Pd/NHC catalysis, the cleavage of the Pd-NHC bond causes deactivation only in the case of aryl chlorides. In the reactions of more active aryl iodides and aryl bromides, NHC-disconnected Pd species, formed as a result of the chemical transformation of Pd/NHC complexes, can provide effective catalysis in the arylation reaction under study. New catalytic systems based on Pd/NHC and Ni/NHC complexes generated in situ from stable imidazolium salts, IPr·HCl and IPr*OMe·HCl, and Pd(OAc)2 (0.1 mol%) or NiCl2Py2 (5 mol%) were developed for the selective α-arylation of methylaryl ketones (Pd-catalysis) and other ketones less prone to aldol-crotonic condensation (Ni-catalysis). The present study has shown that the different effects of the metal-NHC bond cleavage should be taken into account for the efficient choice and optimization of catalytic systems to carry out arylation reaction with various aryl halides.


 Please wait while we load your content...
Please wait while we load your content...