Solvent-induced changes in the reactivity of tricyanate esters undergoing thermal polymerization†
Abstract
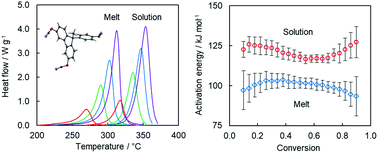
The solvent effect is probed by a comparative study of the tricyanate ester polymerization in the melt and in solution in diphenyl sulfone. Kinetic and mechanistic insights are obtained by a detailed isoconversional analysis of calorimetric data. The rates of both processes are limited by a single reaction of auto-catalytic nature. The reactivity of the tricyanate ester in solution is about five times lower than that in the melt. The diminished reactivity is associated with significantly larger activation energy for polymerization in solution. The increase in the size of the energy barrier is explained by the fact that solvation lowers the reactant energy relative to the energy of the activated complex. Solvation also causes an increase in the pre-exponential factor by increasing the activation entropy of the solution polymerization. The results of the study are expected to contribute to a better understanding of the role of a solvent in the solution polymerization of cyanate esters, which is an important practical route for the synthesis of microporous polytriazines (polycyanurates).


 Please wait while we load your content...
Please wait while we load your content...