Direct access to various C3-substituted sialyl glycal derivatives from 3-iodo-sialyl glycals†
Abstract
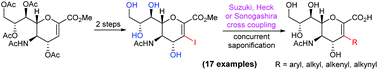
A new and efficient method was developed for the synthesis of C3-substituted sialyl glycals that are useful for novel sialidase inhibitor discovery. This method was based on the cross-coupling reactions of 3-iodo-sialyl glycal methyl ester with boronic acids, alkenes and alkynes to directly introduce various functional groups to the sialyl glycal C3-position. A series of C3-aryl, alkyl, alkenyl, and alkynyl derivatives of sialyl glycal were efficiently and conveniently synthesized for the first time by this method, which has demonstrated its wide application scope.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...