Asymmetric synthesis of functionalized 2,3-dihydrobenzofurans using salicyl N-phosphonyl imines facilitated by group-assisted purification (GAP) chemistry†
Abstract
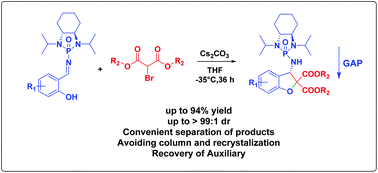
In this work, we present a strategy for the preparation of functionalized 2,3-dihydrobenzofuran derivatives via the Cs2CO3-catalyzed domino annulation of enantiopure chiral salicyl N-phosphonyl imines with bromo malonates, which offers an avenue for the construction of 2,3-dihydrobenzofurans. Nineteen examples were synthesized in impressive chemical yields and diastereoselectivity. The products were purified simply by washing the crude mixtures with hexanes following group-assisted purification chemistry/technology to bypass traditional separation methods which often result in a loss of product. The absolute configuration was unambiguously assigned by X-ray structural analysis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...